Procedure for Obtaining Import Recommendation of Veterinary Medicine, Feed and Veterinary Equipment
| Category | Import |
| Picture |
|
| Summary | Procedure for Importation of Veterinary Medicine, Feed and Equipment issued by Livestock Breeding and Veterinary Department of the Ministry of Agriculture, Livestock and Irrigation |
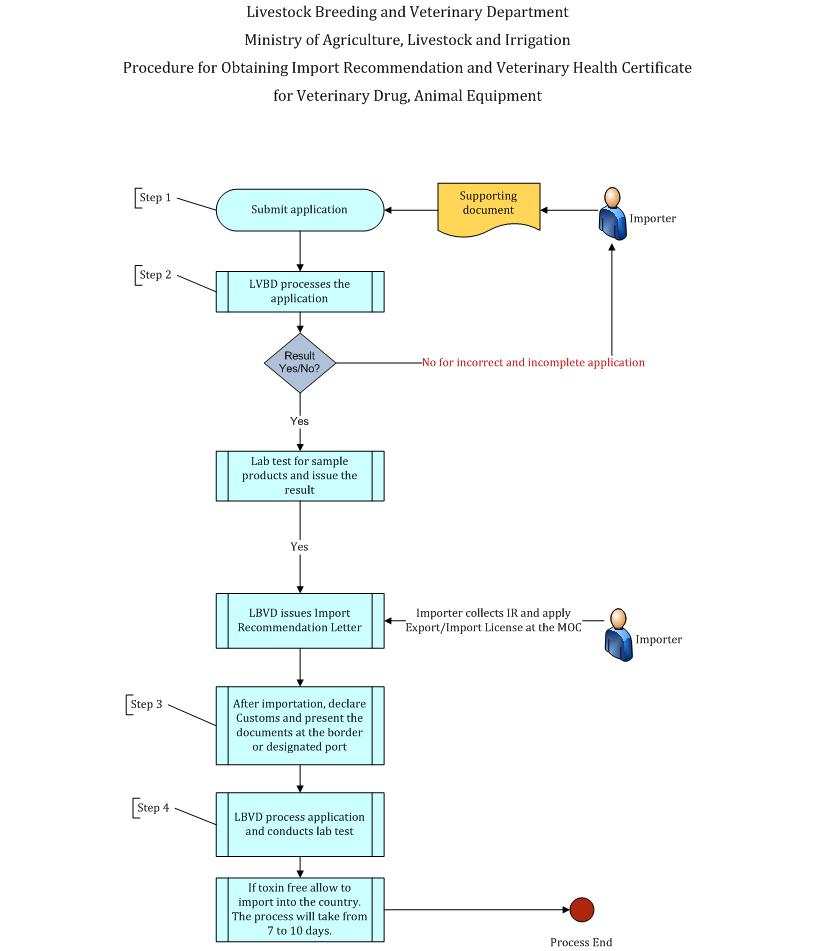
| Description | ivestock Breeding and Veterinary Department Ministry of Agriculture, Livestock and Irrigation Procedure for Importation of Veterinary Medicine, Feed and Equipment Step (1) A person importing veterinary equipment, feed, veterinary medicine into Myanmar shall apply a recommendation certificate, apply to the Livestock Breeding and Veterinary Department (LBVD) along with the following supporting documents.
Step (2) LBVD shall check and review on the completeness of the application and register the documents. For the incomplete and incorrect application, LBVD informs the importer with reason. The importer corrects, completes and resubmit the documents. If application is rejected, the process ends here. The LBVD test the sample products for the assurance of standard quality and free from harmful pathogens or toxins. For the complete and correct application and successful lab test, the LBVD shall issue the import recommendation certificate. After receiving the import recommendation certificate from the LBVD, the importer shall continue to apply the import license at the Ministry of Commerce. Step (3) Upon the arrival to designated port or border gate of Myanmar, the importer shall submit the Import Declaration and relevant documents to the Customs Department and the LBVD for laboratory test via the Myanmar Automated Cargo Clearance System (MACCS). LBVD shall check the sample product of imported veterinary equipment, farm equipment, veterinary medicine signed by authorized Veterinary Officer, including recommendation certificate issued by the LBVD, import license issued by the Department of Trade under the MOC, original Veterinary Health Certificate issued by the Veterinary Authority of exporting country, packing list and bill of lading/Airway bill and PC3 for border trade importation. Step (4) For the successful lab test, the LBVD will provide the result and approval via the MACSS and shall issue the Veterinary Health Certificate (VHC) to the importer. The result will be provided in the Customs Clearance system (MACCS) by the LBVD. The LBVD shall, with the approval of the Ministry establish Inspection Stations in required regions for inspection of imported products. The importer shall pay the laboratory testing fee and Health Certificate fee as determined by the LBVD. Remark- The overall process will take from 7 to 10 days.
|
Forms
The following form/s are used in this procedure.
| Title | Description | Category | Agency |
|---|
